Today, quality management plays an increasingly important role in business organizations. Clients, vendors, and suppliers are demanding higher standards in quality for standard operating procedures, and QMS consulting services help them meet these demands.
Remote audits are becoming more commonplace as, in many businesses, the workforce has become geographically dispersed, and the need for accessible quality management systems continues to increase.
Paper-based and older, centralized software systems are not well equipped to handle many of the challenges of the current business landscape.

For this reason, many organizations are modernizing their approach to quality management by moving their systems to the cloud.
Cloud-based quality management systems offer a flexible, customizable solution to address business and compliance-related needs.
They remove the need for additional in-house infrastructure, cut down on security risks, provide users with the ability to collaborate remotely, and remove the need for patching and upgrading existing systems.
Quality Management Systems
Quality Management Systems (“QMS”) are formalized systems used to document business processes and procedures.
While regulatory bodies determine many quality management system requirements, a successful organization also implements its own protocol for sustaining quality and continual improvement.
ISO1 Standards require that, as a process changes, documentation needs to be updated to reflect those changes.

If documentation becomes outdated, the quality of an organization’s services and products may drop below expectations or organizational requirements.
Maintaining quality process documentation can lead to productivity gains and consistent customer interactions and service, boosting revenue and overall customer satisfaction.
Many organizations have a QMS in place, but the system is outdated—i.e., it has not kept up with the changing business processes of the organization or the market compliance requirements.
Or the QMS is built upon old, inflexible technology that, at best, still functions or, at worst, is no longer supported and does not offer today’s modern collaboration capabilities, support on various devices, or workflow functionality.
A Modern QMS Overview
Common components of a QMS include, but are not limited to, the following:
-
- Document Control
- Nonconformances and CAPA
- Change Requests
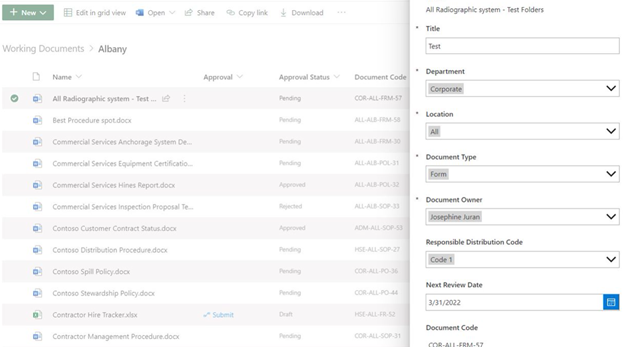
Document Control
Documenting business processes is an essential function of a QMS.
It allows an organization to provide consistent service and results for their customers while increasing productivity by providing a centralized database of business activities.
Simply having the documents is not enough, however.
Maintaining an easy-to-use document library and ensuring that, as processes change, documentation is updated to reflect those changes is imperative.

Following ISO Document Management standards as a guide, you can create a QMS platform using Microsoft SharePoint and the Power Platform—leveraging business tools for which many organizations already have a license today.
A three-stage Document Control system is best to maintain the integrity of the documents and should include:
- Working Documents
- Published Documents
- Obsolete Documents
Working Documents is where process documents are created and allows the author to capture the business process and update the document as needed, as well as access version history.
Once the document is ready for consumption by the business, the author can initiate a review process on the document.
During the review process, members of leadership and any defined approvers or stakeholders verify that the document is correct.
Once approved, a copy of the document is uploaded to the Published documents library.
Publishing a copy allows later updates to the document either as processes change or during a defined review period.
The updated document can then be reviewed, approved, and published again.
Suppose a document is no longer relevant to the business.
In that case, it can be suggested as obsolete.
After approval, the document will be removed from the working and published documents libraries and moved into an Obsolete Documents library for archival purposes.
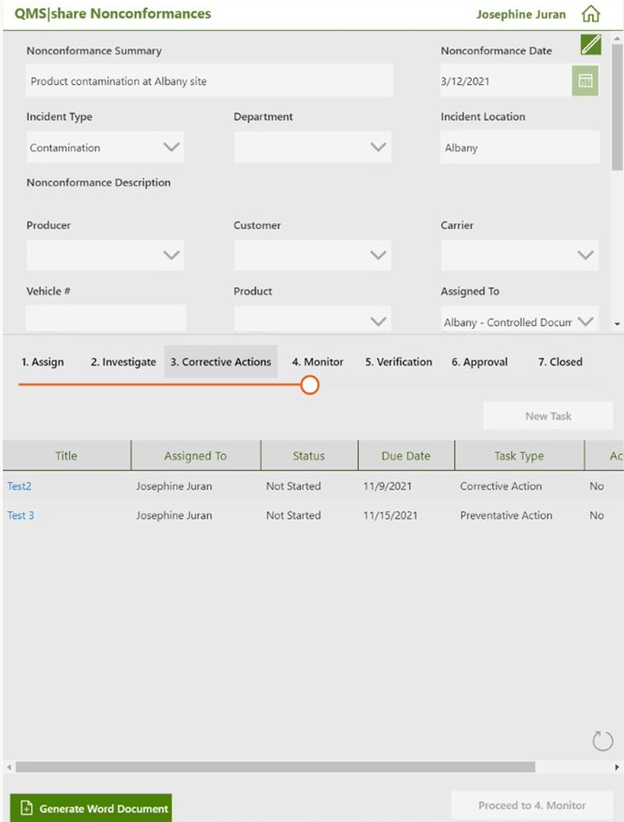
Nonconformances and Corrective and Preventative Actions
Nonconformances are a deviation from existing standards that must be documented and corrected to ensure that the quality requirements of an organization continue to be met. Once one is discovered, it is essential to document the deviation and the recommended Corrective and Preventative Action (CAPA) to address the behavior. An effective QMS can guide users through the process of correcting the nonconformance and through all phases of resolution. These include:
- Short-Term Solutions to mitigate the deviations.
- Root Cause Analysis to identify the nature of the problem.
- Long-Term Solutions to correct the nonconformance.
Each of these phases may require tasks that are connected to each nonconformance and assigned to multiple people and allow for the tracking and completion of each of these tasks.
Critical information must be detailed about each reported nonconformance, including the type of action (Corrective or Preventative) taken, how it was discovered and by whom, and the priority level for the issue. This information can be captured using a tool such as Microsoft Power Apps and stored in a series of related Microsoft SharePoint lists. Additionally, the submitting user can describe the problem in full detail, recommend how to address the problem, and upload supporting document attachments and pictures.
Once all the initial information is adequately captured in the QMS, the solution can guide users assigned to the issue to work through the resolution phases until completion. These phases include implementing short-term solutions, running a root cause analysis, planning and executing a long-term solution to the issue, an approval phase, and a review phase to ensure the nonconformance has been thoroughly addressed.
With a tool like Microsoft Power Automate or Nintex Workflow, assignees and approvers can receive a notification when they have tasks to complete and easily update the requestor on the status of the request. While both platforms integrate with SharePoint, Power Automate typically offers an easier route because most professional SharePoint users also have the full suite of Microsoft 365 at hand.
Change Requests
Changes in a process or procedure are necessary and important, and tracking those changes and ensuring that they will not negatively impact business activities is just as vital.

A QMS can help drive the Change Request and track the Change Orders issued for each request. Keeping all this information in one central, organized location allows for less risk when executing a change.
Leveraging tools like Microsoft Power Apps or Nintex Forms, you can capture Change Request details about the processes, type of equipment, cost, and descriptions and reasons for the requested change and store the information in SharePoint. Change Orders can be created with associated documentation and assigned to technicians. Once the change has been implemented, a review stage allows the Quality Management team to verify the success of the change and confirm completion.
Getting Started with SharePoint QMS
Both SharePoint Online and On-Prem can be used to create and manage a quality management system (QMS) by using its document management features, collaboration, and workflow capabilities. Here are some steps to get started:
- Create a SharePoint site to serve as the hub for your QMS.
- Create document libraries to store and organize your quality-related documents, such as procedures, forms, and records.
- Use SharePoint’s permissions and access controls to ensure that only authorized personnel can view and edit sensitive quality-related documents.
- Use SharePoint’s built-in workflow capabilities to automate and streamline your QMS processes, such as document approvals, reviews, and revisions.
- Create lists to track and manage non-document-based quality data, such as customer complaints, corrective actions, and preventive actions.
- Create dashboards and reports to easily monitor and track key performance indicators (KPIs) related to your QMS.
- Create a communication system for the internal and external stakeholders using SharePoint features like Team site, communication site, News, etc.
- Use SharePoint’s integration capabilities to connect your QMS with other systems, such as enterprise resource planning (ERP) and customer relationship management (CRM) systems, to ensure data consistency and accuracy.
Summary
Modernizing legacy Quality Management Systems by adopting SharePoint can significantly enhance the productivity of your organization and ease many of the challenges of our rapidly changing working environments.

It can allow for secure remote access by auditors and users alike, accommodating hybrid work environments and geographically diverse workforces.
It provides flexibility in how the quality process is maintained and improved over time by using tools that are continually updated and enhanced.
Finally, it can be adapted to provide greater collaboration between users in multiple departments and organizations to improve quality processes.
Whether you are searching for a project-specific solution or a company-wide evaluation of your company’s QMS, our QMS Consulting Services can guide you through the process.
Contact us today to talk to one of our QMS experts and learn about the solution we built leveraging Microsoft SharePoint and how it can help your business!
This Abel insight was written by Abel Solutions SharePoint Consultant, Chris Wirkus.
1International Organization for Standards (ISO) – ISO is an independent international organization that consists of 167 national standards bodies. The goal of ISO is to provide solutions to global challenges particularly in the fields of manufacturing and technology.




