As industry-leading SharePoint consultants, we provide strategy, create and implement solutions, and offer ongoing support across the SharePoint platform.
[vc_row][vc_column][vc_column_text]Organizations that follow ISO standards or any Quality Management standard need a way to track and analyze problems, follow a defined process to determine what caused the problem (root cause analysis), and then create and execute a plan to prevent it from occurring again. Microsoft SharePoint (on-premises or Office 365) can be leveraged for your Quality Management System to facilitate this process from beginning to end.
Overview
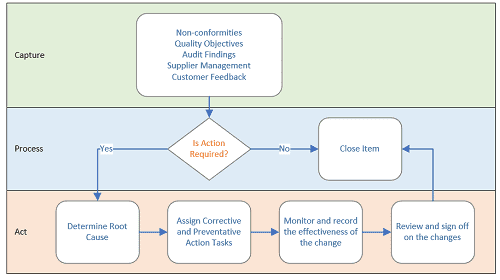
From a high level, we want to accomplish the following:
- Capture items that need to be tracked.
- Process those items and determine if further action is necessary.
- Act to make the necessary changes, monitor those changes, and record the results.
Figure 1 Overview of the Quality Management Process
Capture
There are many types of items that need to be captured and tracked within your organization from a Quality Management perspective. Here are some common examples of items we often see:
- Non-conformities
A deviation from a standard or expectation. Non-conformities are typically classified as major or minor deviations. - Audit Findings
The results of any internal or external audits. - Quality Objectives
Written goal that may be directly associated with your organizations Quality Policy. It may include things like “Improve delivery time by x%”, or “Reduce defects by x%”. - Supplier Management
What suppliers does your organization are certified for your organization to use? There may also be non-conformities associated with specific suppliers that you will want to track. - Customer Feedback
Positive or negative feedback from customers.
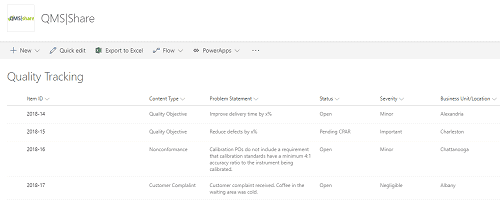
All these items can easily be tracked in SharePoint (on-premises or Office 365) using lists or libraries. Each item can be tagged with specific metadata needed so that it can be found quickly, and reports can be generated from the data that is collected.
Figure 2 Here is an example of a Quality Tracking list in SharePoint
Process
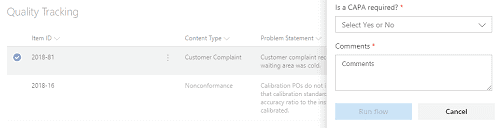
Now that we have data being captured, we will want to process it. Someone would review the open items and determine if further action is needed or not. This is when you can initiate a CAPA (Corrective and Preventative Action). The CAPA, also known as CPAR or sometimes just Corrective Action) is a set of actions taken to eliminate the causes of a non-conformity or other type of problem.
In SharePoint, we can automatically create a new CAPA based on any item that was captured. This eliminates the need to retype data (which can lead to errors) and provides end-to-end tracking since the original problem is tied directly to the CAPA item.
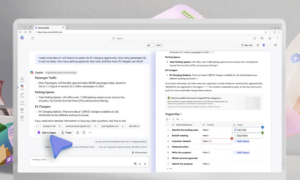
Figure 3 Example showing how to trigger a CAPA from a captured item
Act
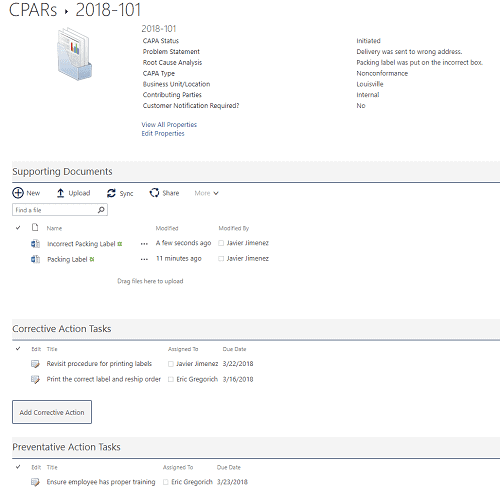
Within the CAPA, you can gather all the details, including root cause and contributing parties; supporting documents and images; and tasks for assignment. When all the tasks and changes have been completed, you can monitor the results and then record the effectiveness of the changes to determine if the CAPA was successful.
Figure 4 Example showing a CAPA details page
As a Microsoft Gold Partner, we offer Microsoft 365-based solutions to help businesses optimize their quality management systems.
If you’d like to learn more about how our SharePoint Solutions quality management solutions can transform your business, visit our SharePoint Consulting page.
Conclusion
At Abel Solutions, we have years of experience building Quality Management solutions. Moving from the file system and Excel spreadsheets into SharePoint provides greater visibility and control of your data, and the workflow capabilities can manage your business process around this data ensuring that your organization is following a consistent process.
To simplify implementation, we have developed QMS|share, a solution accelerator designed to allow organizations to move their Quality Management processes into SharePoint quickly and efficiently.
This tip written by Abel Solutions Senior Solutions Architect Eric Gregorich.[/vc_column_text][/vc_column][/vc_row]